Customer Service
Q&A
Huons Meditech Showcases at Arab Health 2025 in Dubai
Huons Meditech Showcases at Arab Health 2025 in Dubai

Huons Meditech, a specialized medical device company under the Huons Group, is actively expanding its global influence through participation in international exhibitions and local promotional activities. The company announced on January 31 that it participated in Arab Health 2025, the largest medical device exhibition in the Middle East, held recently in Dubai, UAE.
Celebrating its 50th anniversary this year, Arab Health has been a cornerstone event in the MENA (Middle East and North Africa) region since its inception in 1975. The exhibition brings together around 4,000 exhibitors and over 60,000 visitors annually, offering networking opportunities and fostering advancements in healthcare across various sectors, including medical devices, pharmaceuticals, and healthcare services.
At the exhibition, Huons Meditech showcased a diverse portfolio of medical devices, drawing significant attention from buyers with the introduction of its kidney stone fragmentation device, ASADAL-M1, which is set to enter the Middle Eastern market.
The ASADAL-M1 has received both FDA and CE certifications and utilizes magnetic and extracorporeal shock wave technology to provide a high stone fragmentation rate with enhanced safety. As a non-invasive treatment, it offers a shorter procedure time compared to traditional surgical methods, allowing for same-day treatment and discharge, a key benefit for patients.
In addition, Huons Meditech highlighted its popular drug delivery device, Dermashine Pro, which has surpassed cumulative sales of 20,000 units globally. The device received considerable attention from visitors, reinforcing the company’s strong position in the global aesthetic market. As the demand for polymer-based skin boosters continues to rise, the need for precise drug delivery devices and specialized needles is also increasing. To meet market demands, Huons Meditech is continuously developing new products, including next-generation versions of Dermashine Pro and a variety of multi-needles designed for polymer-based booster injections.
Jin Suk Lee, CEO of Huons Meditech, stated, “As we aim to expand in the global medical device market, we are focusing on overseas marketing strategies, including participation in international conferences and exhibitions to enhance our competitiveness. With ongoing efforts to obtain CE and FDA certifications for various products, including our lithotripsy devices URO-UEMXD and EMXD, as well as Dermashine, we are committed to becoming a leading global medical device company within the next three years.”
-

 Huons Meditech drives global growth, participating in medical device symposiums in AU&US
Huons Meditech drives global growth, participating in medical device symposiums in AU&US
-

 Huons Meditech to showcase at Healthcare Trade Fair ‘Hospitalar 2025’ in Brazil
Huons Meditech to showcase at Healthcare Trade Fair ‘Hospitalar 2025’ in Brazil
-

 Huons Meditech receives Taiwan FDA approval for gynecological drug delivery system 'Jill'SOF'
Huons Meditech receives Taiwan FDA approval for gynecological drug delivery system 'Jill'SOF'
-

 Huons Group participates ‘Dubai Derma 2025’ for efforts to maximize sales
Huons Group participates ‘Dubai Derma 2025’ for efforts to maximize sales
-
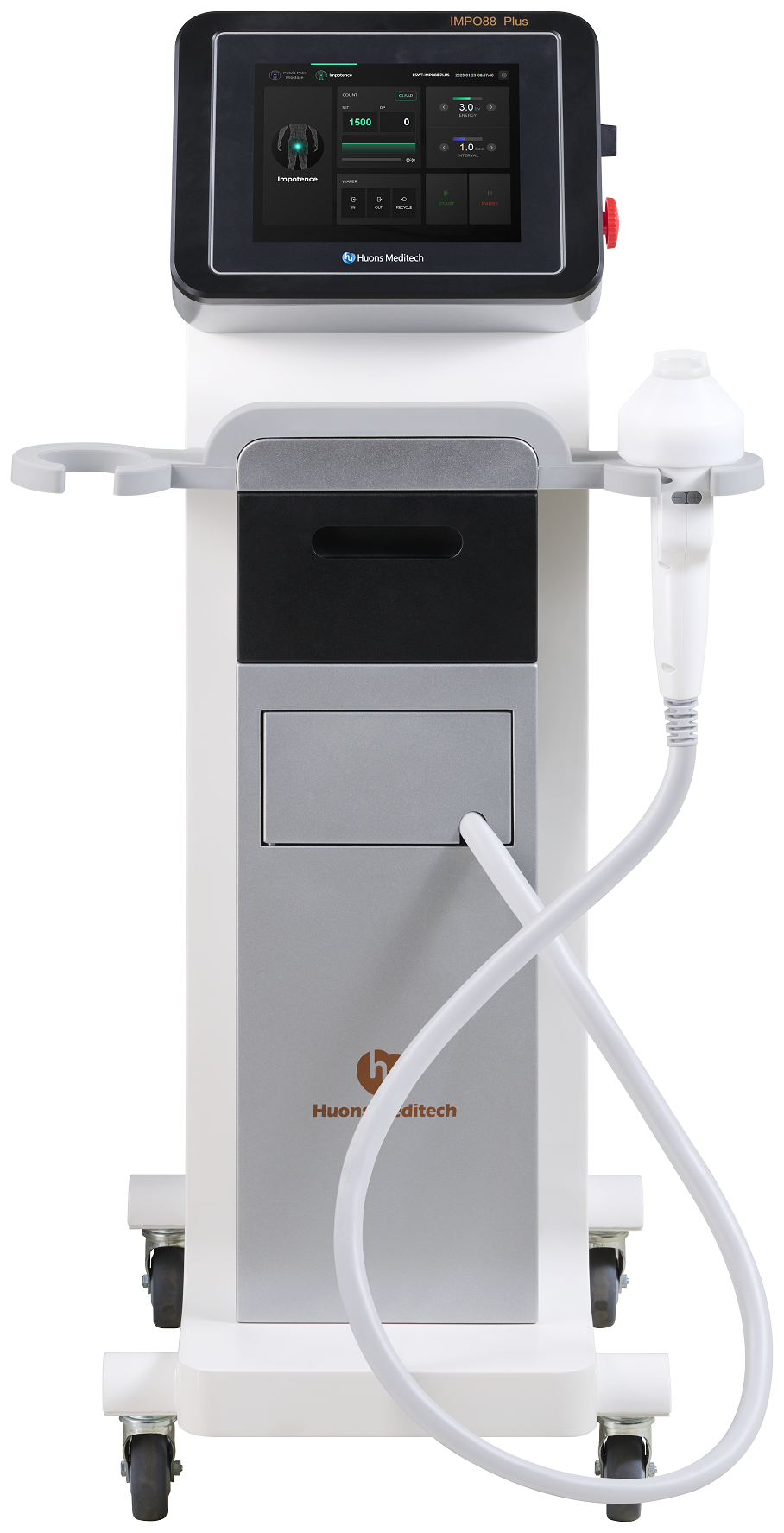
 Huons Meditech officially launches Extracorporeal Shock Wave Therapy device ‘IMPO88 Plus’ for Erectile Dysfunction - Proven effect on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Treatment -
Huons Meditech officially launches Extracorporeal Shock Wave Therapy device ‘IMPO88 Plus’ for Erectile Dysfunction - Proven effect on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Treatment -
-

 Huons Meditech Showcases Innovative Medical Devices at ‘KIMES 2025’
Huons Meditech Showcases Innovative Medical Devices at ‘KIMES 2025’
-

 Huons Meditech Showcases at Arab Health 2025 in Dubai
Huons Meditech Showcases at Arab Health 2025 in Dubai
-
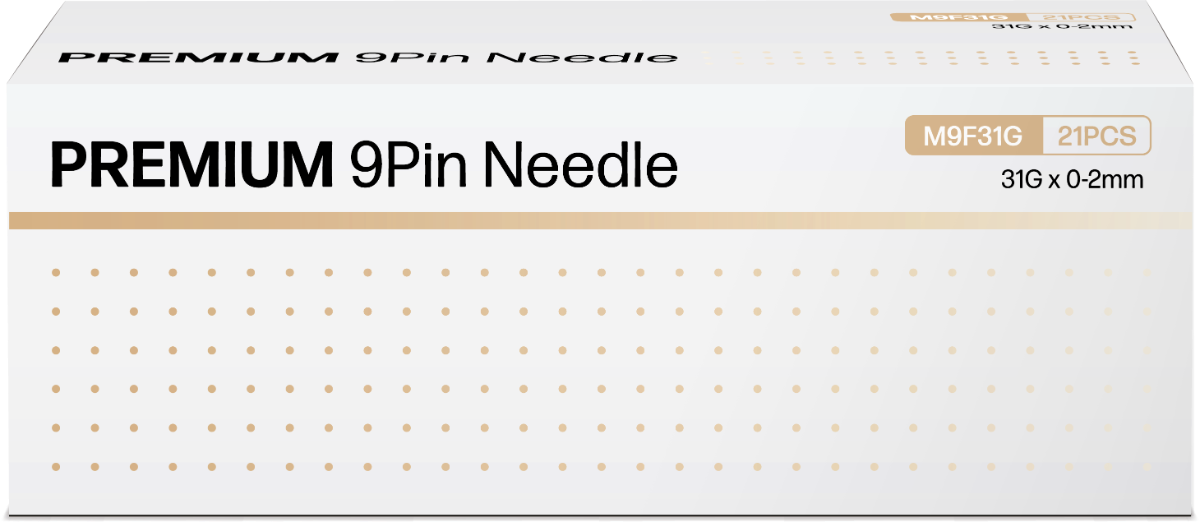
 Huons Meditech Launches ‘Premium 9-Pin Needle 31G’ for Skin Booster Treatments
Huons Meditech Launches ‘Premium 9-Pin Needle 31G’ for Skin Booster Treatments
-

 Huons Meditech Showcases Expertise at the ‘Society of Female Functional Urology and Sexual Medicine International Seminar’
Huons Meditech Showcases Expertise at the ‘Society of Female Functional Urology and Sexual Medicine International Seminar’
-

 Huons Meditech Accelerates Overseas Marketing
Huons Meditech Accelerates Overseas Marketing
-

 Huons Meditech Showcases Key Products at FIME 2024 in Florida
Huons Meditech Showcases Key Products at FIME 2024 in Florida
-

 Huons Meditech Targets South American Market at 'Hospitalar 2024' Exhibition in Brazil
Huons Meditech Targets South American Market at 'Hospitalar 2024' Exhibition in Brazil








