Customer Service
Q&A
Huons Meditech Showcases Key Products at FIME 2024 in Florida
Huons Meditech Showcases Key Products at FIME 2024 in Florida
- Innovative Devices Like the ASADAL-M1, Lithotripter Draw Significant Interests -

▲ held at the Miami Beach Convention Center. The company’s booth, where representatives engaged with attendees, showcased some of its leading medical devices, capturing considerable attention.
Huons Meditech (CEO Jin Suk Lee) announced on June 24 that it participated in FIME 2024, the largest medical device exhibition in the United States, held from June 19 to 21.
At the exhibition, Huons Meditech unveiled the ASADAL-M1 lithotripter, designed for the fragmentation of kidney stones, and the Huen Single endoscope washer/disinfector in the CSSD segment. In the aesthetic category, the company promoted the DermaShine Pro, a mesotherapy injector utilizing negative pressure. These offerings drew significant interests from international buyers and attendees.
The ASADAL-M1, certified by the U.S. Food and Drug Administration (FDA) and the European Medical Device Regulation (CE), employs electro-magnetic extracorporeal shockwave (ESWL) technology to ensure safe and efficient lithotripsy. The non-invasive ESWL procedure, in contrast to traditional surgical stone removal, boasts a quick procedure time and allows for same-day treatment and discharge.
The Huen Single is an automatic endoscope washer/disinfector using a single-use peracetic acid (PAA) disinfectant solution, Sco-Single. It inhibits biofilm formation on both internal and external surfaces of endoscopes and sterilizes all pathogenic microorganisms, including spores, within five minutes. Unlike many endoscope disinfectors that reuse disinfectant solutions, increasing the risk of cross-contamination, the Huen Single uses a single-use disinfectant, eliminating the risk. It also features Clean-in-Place (CIP) functionality and a photocatalytic filter that purifies peracetic acid gases and odors, enhancing device durability and user safety, which attracted high interest from attendees.
The DermaShine Pro, the latest model in the highly successful DermaShine series, features an upgraded pressure-sensing injection system that allows for precise drug infusion and speed settings. Compatible with various syringes (32G, 34G, and 9-pin sterile needles), the device is expected for U.S FDA approval by the second half of next year, leading to numerous inquiries from attendees.
Jin Suk Lee, CEO of Huons Meditech, remarked, "We are strengthening partnerships to expand into global markets through participation in major exhibitions," adding, "We will continue to introduce our innovative medical devices, developed with proprietary technology, at exhibitions to increase buyer interest and strive to establish ourselves as a leading global medical device company."
-

 Huons Meditech offers a new paradigm for ESWT in urology
Huons Meditech offers a new paradigm for ESWT in urology
-

 Huons Meditech drives global growth, participating in medical device symposiums in AU&US
Huons Meditech drives global growth, participating in medical device symposiums in AU&US
-

 Huons Meditech to showcase at Healthcare Trade Fair ‘Hospitalar 2025’ in Brazil
Huons Meditech to showcase at Healthcare Trade Fair ‘Hospitalar 2025’ in Brazil
-

 Huons Meditech receives Taiwan FDA approval for gynecological drug delivery system 'Jill'SOF'
Huons Meditech receives Taiwan FDA approval for gynecological drug delivery system 'Jill'SOF'
-

 Huons Group participates ‘Dubai Derma 2025’ for efforts to maximize sales
Huons Group participates ‘Dubai Derma 2025’ for efforts to maximize sales
-
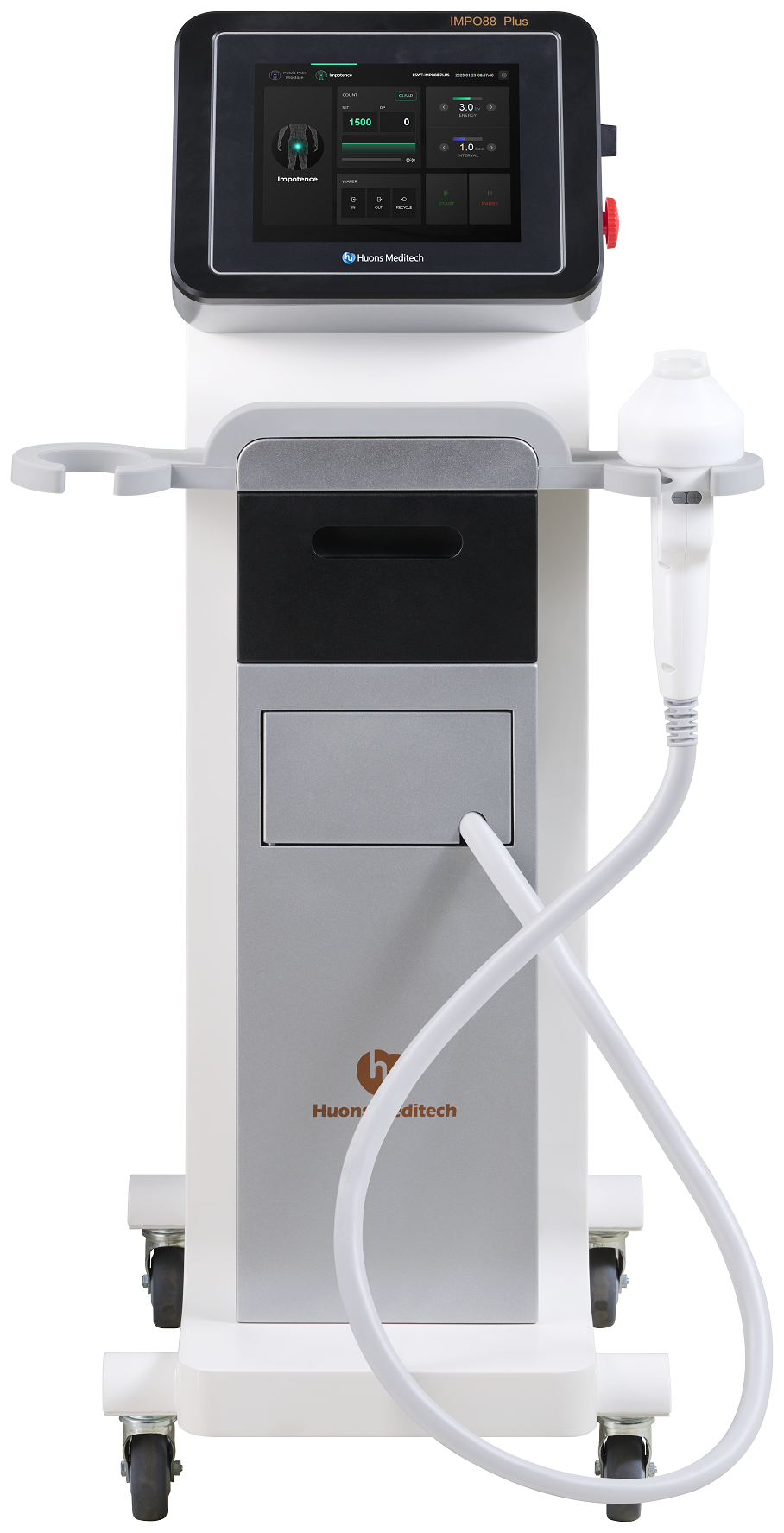
 Huons Meditech officially launches Extracorporeal Shock Wave Therapy device ‘IMPO88 Plus’ for Erectile Dysfunction - Proven effect on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Treatment -
Huons Meditech officially launches Extracorporeal Shock Wave Therapy device ‘IMPO88 Plus’ for Erectile Dysfunction - Proven effect on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Treatment -
-

 Huons Meditech Showcases Innovative Medical Devices at ‘KIMES 2025’
Huons Meditech Showcases Innovative Medical Devices at ‘KIMES 2025’
-

 Huons Meditech Showcases at Arab Health 2025 in Dubai
Huons Meditech Showcases at Arab Health 2025 in Dubai
-
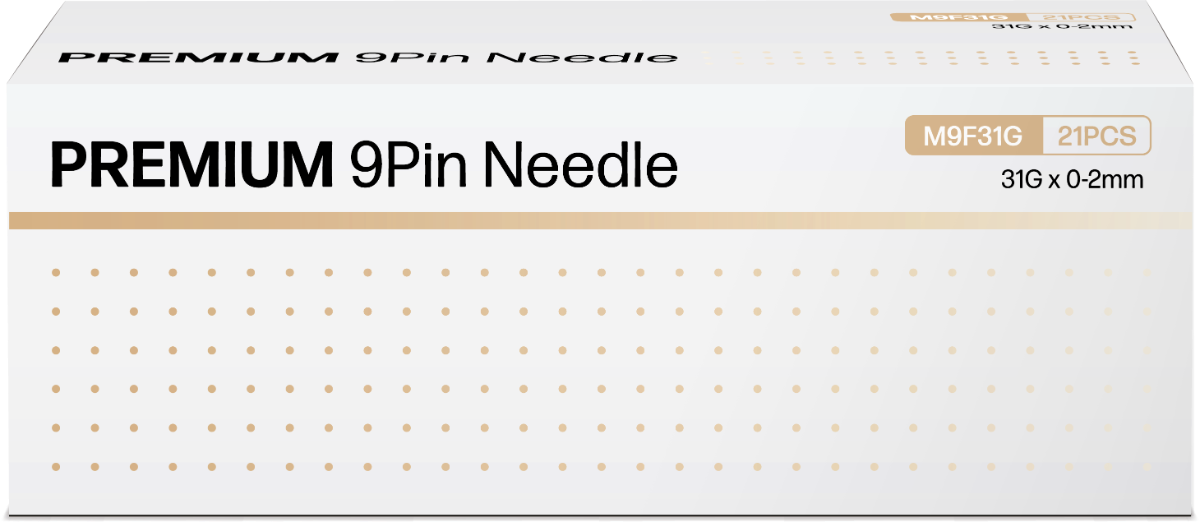
 Huons Meditech Launches ‘Premium 9-Pin Needle 31G’ for Skin Booster Treatments
Huons Meditech Launches ‘Premium 9-Pin Needle 31G’ for Skin Booster Treatments
-

 Huons Meditech Showcases Expertise at the ‘Society of Female Functional Urology and Sexual Medicine International Seminar’
Huons Meditech Showcases Expertise at the ‘Society of Female Functional Urology and Sexual Medicine International Seminar’
-

 Huons Meditech Accelerates Overseas Marketing
Huons Meditech Accelerates Overseas Marketing
-

 Huons Meditech Showcases Key Products at FIME 2024 in Florida
Huons Meditech Showcases Key Products at FIME 2024 in Florida








