Customer Service
Q&A
Huons Meditech Makes Strides with 'Dermashine Pro' as TFDA Greenlights Taiwanese Market Entry
|
Huons Meditech Makes Strides with 'Dermashine Pro' as TFDA Greenlights Taiwanese Market Entry
▲ Huons Meditech Co., Ltd.'s medical equipment for water injection procedures 'Dermashine Pro' In a groundbreaking development, Huons Meditech proudly announces its latest achievement on August 16th – the official approval from the Taiwan Food and Drug Administration (TFDA) for the innovative 'Dermashine Pro.' This cutting-edge digital infusion pump, accompanied by patented 9-pin multi-needles, is set to revolutionize the aesthetic landscape in Taiwan and beyond. The debut of 'Dermashine Pro' marks a pivotal moment for Huons Meditech as it unveils the upgraded version of the highly acclaimed first-generation 'Dermashine,' which amassed a remarkable 20,000 units sold globally since its domestic launch in 2014. This bold move solidifies Huons Meditech's standing as a frontrunner in the realm of dermatological digital infusion systems, maintaining a steadfast reputation in the dynamic global aesthetic market. 'Dermashine Pro' isn't merely a device; it's a sophisticated aesthetic marvel designed to enhance skincare regimens. Through its patented 9-pin multi-needle technology, this medical wonder administers a precision injection of high-molecular-weight hyaluronic acid, exemplified by the exceptional 'EllaVie Balance,' ensuring a flawless experience. The pressure-sensitive automatic injection system raises the bar for skincare excellence. Since obtaining the coveted China Food and Drug Administration (CFDA) approval for the first-generation 'Dermashine' in 2014, Huons Meditech has consistently expanded its global presence. The cutting-edge 'Dermashine Pro' has garnered acclaim for its unwavering quality, safety, durability, and performance. Having already secured the prestigious European CE mark, the device is also on track to obtain the highly sought-after U.S. FDA's approval. The TFDA's seal of approval holds immense significance in the world of medical devices and pharmaceuticals. TFDA is the authoritative body overseeing the manufacturing, supervision, and distribution of these products in Taiwan. Products endorsed by TFDA stand as paragons of quality and reliability, facilitating streamlined approval processes in global expansion endeavors. Commenting on this monumental achievement, Huons Meditech stated, "Building upon our resounding success in the global market, the TFDA's approval for 'Dermashine Pro' opens doors to a new era. With plans to venture into overseas aesthetic markets, including the U.S. and Canadian markets, and fostering strategic alliances with esteemed global aesthetic companies, we are poised to redefine the global aesthetic landscape." Huons Meditech's stride into the Taiwanese market signifies a significant leap forward, bearing the promise of transforming skincare routines worldwide. The 'Dermashine Pro' stands as a testament to innovation, quality, and a commitment to enhancing beauty experiences globally. |
-

 Huons Meditech offers a new paradigm for ESWT in urology
Huons Meditech offers a new paradigm for ESWT in urology
-

 Huons Meditech drives global growth, participating in medical device symposiums in AU&US
Huons Meditech drives global growth, participating in medical device symposiums in AU&US
-

 Huons Meditech to showcase at Healthcare Trade Fair ‘Hospitalar 2025’ in Brazil
Huons Meditech to showcase at Healthcare Trade Fair ‘Hospitalar 2025’ in Brazil
-

 Huons Meditech receives Taiwan FDA approval for gynecological drug delivery system 'Jill'SOF'
Huons Meditech receives Taiwan FDA approval for gynecological drug delivery system 'Jill'SOF'
-

 Huons Group participates ‘Dubai Derma 2025’ for efforts to maximize sales
Huons Group participates ‘Dubai Derma 2025’ for efforts to maximize sales
-
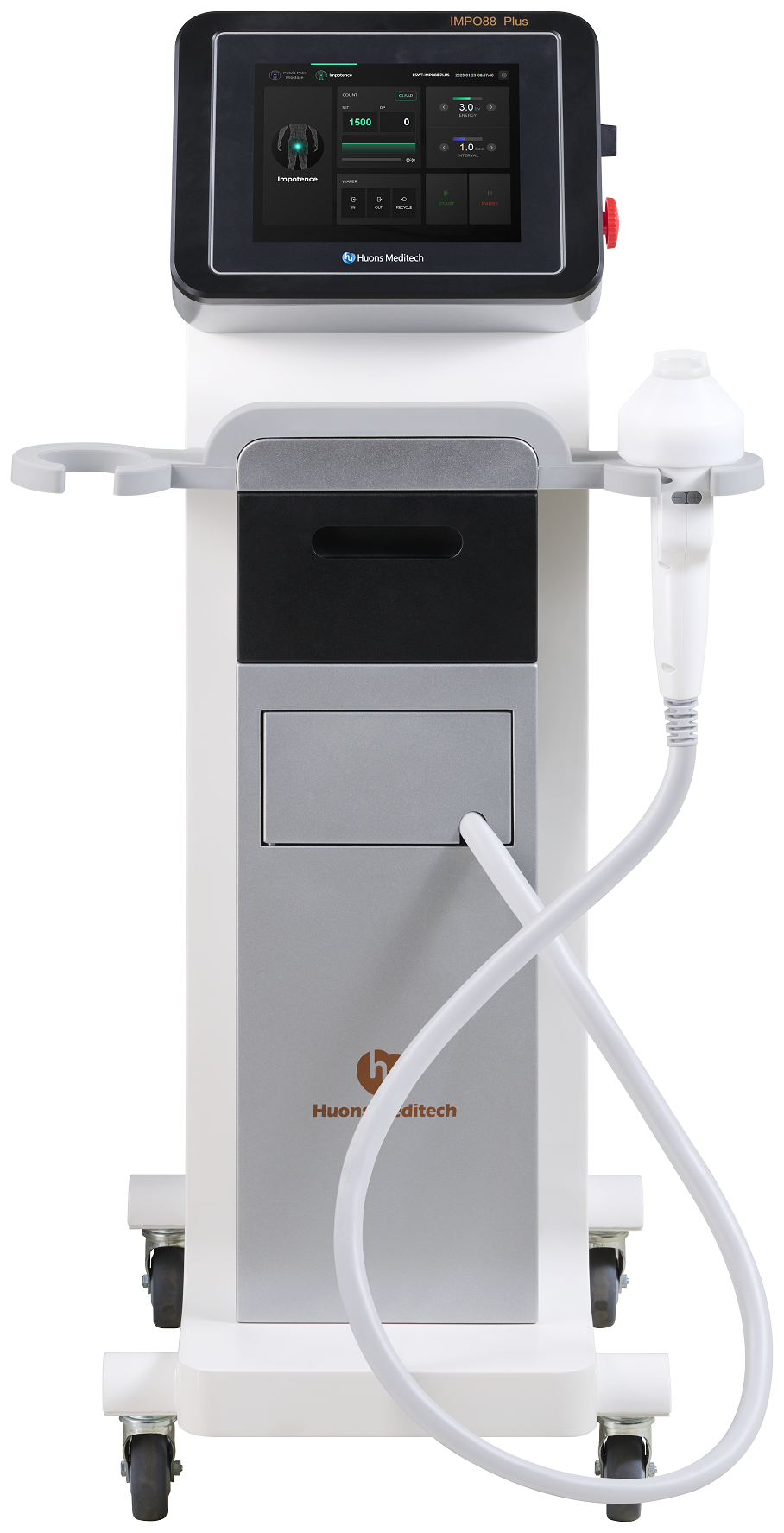
 Huons Meditech officially launches Extracorporeal Shock Wave Therapy device ‘IMPO88 Plus’ for Erectile Dysfunction - Proven effect on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Treatment -
Huons Meditech officially launches Extracorporeal Shock Wave Therapy device ‘IMPO88 Plus’ for Erectile Dysfunction - Proven effect on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Treatment -
-

 Huons Meditech Showcases Innovative Medical Devices at ‘KIMES 2025’
Huons Meditech Showcases Innovative Medical Devices at ‘KIMES 2025’
-

 Huons Meditech Showcases at Arab Health 2025 in Dubai
Huons Meditech Showcases at Arab Health 2025 in Dubai
-
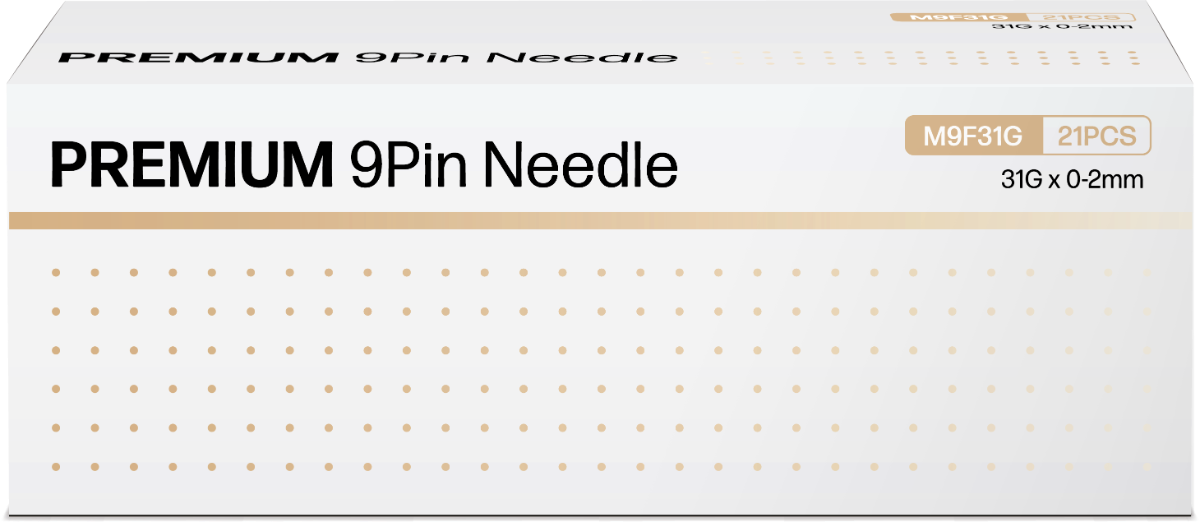
 Huons Meditech Launches ‘Premium 9-Pin Needle 31G’ for Skin Booster Treatments
Huons Meditech Launches ‘Premium 9-Pin Needle 31G’ for Skin Booster Treatments
-

 Huons Meditech Showcases Expertise at the ‘Society of Female Functional Urology and Sexual Medicine International Seminar’
Huons Meditech Showcases Expertise at the ‘Society of Female Functional Urology and Sexual Medicine International Seminar’
-

 Huons Meditech Accelerates Overseas Marketing
Huons Meditech Accelerates Overseas Marketing
-

 Huons Meditech Showcases Key Products at FIME 2024 in Florida
Huons Meditech Showcases Key Products at FIME 2024 in Florida









